Anal Cancer Screening Guidelines for PLHIV
Last updated: November 2024
Key recommendations of the ASHM Anal Cancer Screening Guidelines Committee
In determining specific recommendations for screening to prevent anal squamous cell cancer (ASCC) in PLHIV in Australia, we must acknowledge that the evidence base is limited. However, based on current evidence we recommend:
- Gay, bisexual and other men who have sex with men (GBM) and trans women (TW) LHIV 35 years and older should be offered screening 10–17–26
- Cis-women, trans men and other cis-men (not GBM) LHIV 45 years and older should be offered screening 10–17–26
- The screening modality should be primary HRHPV testing with cytology triage17–31–38 (Figure 2)
- Screening should be repeated every 3 years for those who screen negative 17–64 (based on screening women LHIV for cervical cancer – every 3 years)
- Screening should be discontinued, with shared decision-making, at age 75 years and in individuals with two consecutive negative screening visits who are not currently sexually active 17–18–20–64 (only screen to 74 years for cervical cancer, with some caveats)
All anal cancer screening should include annual DARE, examination of the peri-anal region and a thorough medical history. The history should
Include sexual behavioural history, as anal sexual activity may not have been previously disclosed.
Identify other potential risk activities (such as smoking) and other factors that may contribute to immunosuppression (such as certain drugs)
As anal cancer screening and HRA capacity will be limited as screening commences, clinicians should prioritise screening of PLHIV based on the following factors that are known risk factors for anal cancer:
- Older age
- CD4 nadir of 200 cells/µL or less
- Current smoker
- History of anal sexual activity
- Current anal symptoms of pain, change in anal bleeding or lump*
* Patients with an anal lump should also have a surgical review
An expert group should be established to develop pathways for the management of screen-detected abnormalities in PLHIV based on current and evolving evidence.
Figure 2. Proposed screening algorithm with primary extended genotyping HPV testing
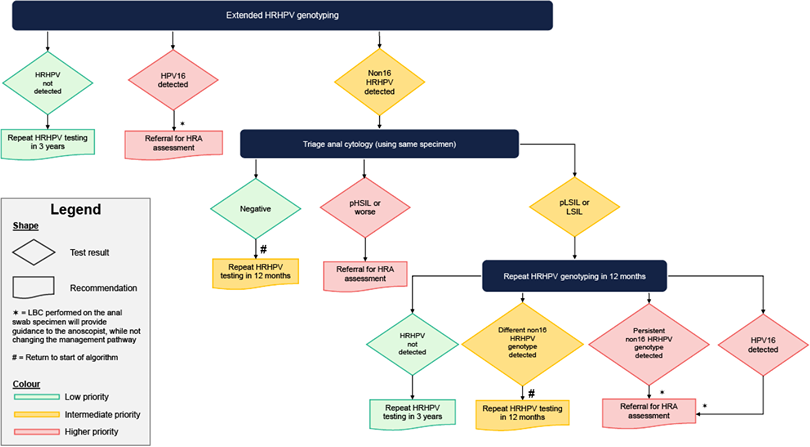
Definitions:
LSIL: low-grade squamous intraepithelial lesion
pLSIL: possible low-grade squamous intraepithelial lesion
HSIL: high-grade squamous intraepithelial lesion
pHSIL: possible high-grade squamous intraepithelial lesion
Resources
Legal
About Us
Contact Us
ASHM Head Office – Sydney
Level 3, 160 Clarence Street Sydney, NSW 2000
Tel: (+61) 02 8204 0700 Fax: (+61) 02 8204 0782
![]()
![]() Acknowledgement of Country
Acknowledgement of Country
ASHM acknowledges the Traditional Owners of Country across the various lands on which we live and work. We recognise Aboriginal and Torres Strait Islander peoples’ continuing connection to land, water, and community and we pay our respects to Elders past and present. ASHM acknowledges Sovereignty in this country has never been ceded. It always was, and always will be, Aboriginal land.
ASHM Health | ABN 48 264 545 457 | CFN 17788 | Copyright © 2024 ASHM

